Handling Adverse Events on Social Media
By developing and implementing solutions for handling adverse events in social media, your company has the opportunity to leverage key information from the largest focus group in the world.
In my previous column, I discussed how social media can be viewed as a “perpetual focus group.” The sheer and ever-expanding volume of these conversations represent a virtually infinite set of data that can be transformed into valuable information. All of this information — even Adverse Events (AEs) and Product Quality Complaints (PQCs) — can provide insights that power decisions and support action.
The role of adverse events on social media
Social media, like any other media, is a source of AEs and PQCs. Given the immense volume of tweets, Facebook status updates, Instagram photos, blog posts, and conversations on forums, it is to be expected that while people are sharing their health experiences, AEs and PQCs are bound to be mentioned. These can occur either intentionally (for example, “I took Drug Rx and I woke up to a rash on my leg”) or incidentally (“I’m so grateful for the nurses who took such good care of me when I was in the hospital after my car accident. Also, Drug Rx has been working well for me.”).
Since the early days of social media, there have been two opposing schools of thought on the best ways to handle AEs on social media. One school of thought views AEs as an absolute obstacle to any pharmaceutical company getting involved in social media. The other school does not view AEs as a problem, but rather, in my opinion, projects a nonchalant attitude about the nuances of social conversations and the identification of AEs.
I am not a student of either school of thought. Based on my professional experience within clinical settings and my knowledge of drug safety, I believe the best stance is to acknowledge the challenges that AEs present while, at the same time, to develop and implement practical day-to-day solutions — systems that ensure proper identification and triaging of AEs, as well as the ability to leverage AEs for analytics and insights.
How to monitor and categorize adverse events
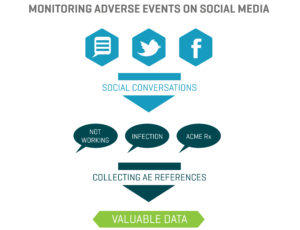 Fundamentally, AEs can yield useful information when monitored closely, safely, and properly. Over a period of several months, for instance, thousands of AE posts can be classified according to an objective-based process. Then, identified and triaged appropriately, these AEs can be classified into categories.
Fundamentally, AEs can yield useful information when monitored closely, safely, and properly. Over a period of several months, for instance, thousands of AE posts can be classified according to an objective-based process. Then, identified and triaged appropriately, these AEs can be classified into categories.
Let’s explore one example: “Drug Rx has been a life-changer for me. So far, it’s been doing what my doctor says it’s supposed to do. Occasionally, I get a little rash on my right arm, but other than that, I’m truly grateful.”
In this scenario, we have an AE (assuming we can identify the person reporting it). We also have references that can be classified. “It’s doing what my doctor says it’s supposed to do” could be classified as Effectiveness, and “a little rash” could be classified as, say, Immunity. Over time, these AEs can help companies better understand conversations. At the end of the set time period, a company may find that 50% of AEs were Immunity-related, 25% were Cardiovascular, 15% were Respiratory, and so on — providing valuable information for understanding the adverse side effects of a particular agent.
Furthermore, if these posts were also categorized by other data, such as the social media outlet, the topics discussed and the behaviors expressed, the aggregate data offers information beyond purely clinical, perhaps even helping to inform a company’s messaging or marketing strategy.
Establishing well-defined protocols for success
The above example illustrates the basic concept of leveraging social media AE data. In essence, the close monitoring and careful categorization of social media AE data can transform a seemingly complex and vast landscape into a navigable road. But that’s not to ignore the challenge of scalability. The best approach is to start small, evaluate results and evolve incrementally.
Companies need to plan for how they will monitor social media for AEs, because the greatest pitfall is missing AEs altogether and risking patient safety. Training staff in the identification of AEs and the enterprise’s triaging protocol is crucial. In my company’s training program, our agents receive training in the nuances of social media, in addition to traditional AE and PQC training, to ensure they do not miss AEs. After identification, the documentation and communication protocols need to be established and clearly understood by all team members.
The first step of merely identifying AEs can be challenging within itself, as information related to AEs or potential AEs are often ambiguous or sparse, especially due to the word or character limitations on certain social media platforms, such as Twitter. Furthermore, a company cannot publicly respond to a tweet that simply says, “My inhaler isn’t working” by asking, “What do you mean by working”? So the company is left wondering, is this an AE (lack of efficacy), a PQC (a malfunction), both an AE and a PQC, and/or an opportunity for patient education? In the inhaler example, the company could respond with, “We’re interested in learning more. Please contact us at 800-555-5555.” This is where the pharmaceutical company’s ability to interact publicly in social reaches its limit, but still presents an opportunity to demonstrate a caring, human voice, while adhering to regulations. Training must include steps for evaluating an AE and how to extract the key data.
Exploring ethical implications
Because the general principles of monitoring for AEs also apply to social media, compliance with FDA regulations is, for the most part, straightforward. Yet beyond the regulatory responsibilities of AEs, ethical issues arise. For example, it is inevitable that companies may stumble upon mentions of a competitor’s agent with a potential AE, either on their own branded properties or within the broad social media space. Companies are not required to report another company’s AEs to the FDA, but should they? Well-established processes, understood by all within a company, can help guide these and other ethical decisions.
Pharmaceutical companies are committed to patient safety, product safety, and effectiveness. By investing in and developing resources to ethically and efficiently manage AEs on social media, companies can transform the fear of these AEs into mutually beneficial results. In the long run, better understanding of all AEs — social media or otherwise — can help expand the knowledge of drug researchers, providers, and marketers; advance the industry; and improve the well-being of the patients we serve.