It’s probably safe to say that you’ve been to the doctor at least once in your adult life. So you can probably relate to this:
You’re sitting on the exam room table in your physician’s office. In your head, there’s a lengthy list of questions related to the reason for your visit – an aching back, perhaps, or persistent heartburn, maybe a minor sprain – and you’re hoping you don’t forget any of them. Your doctor walks into the exam room after what feels like an eternity, even though it’s probably only been 10 minutes, and asks, “How are you today?”
“I’m fine,” you answer.
You’re not alone! Doctors say this is a common scenario … and one that makes effectively treating patients all the more challenging. “I’m fine” has become a default response.
One of the most important interactions individuals can have is about their health. But empowering patients, educating them to take control of their health – avoiding the default “I’m fine” answer – is one of the greatest challenges DTC marketers face. In fact, it’s one of the greatest communication challenges in healthcare, period.
It’s also one of the greatest opportunities. So how can you overcome that challenge? Where do you start?
In my opinion, the most effective way to overcome communication challenges starts in the exam room. And it’s so critical to the patient, the physician and the pharmaceutical marketer to get that interaction right.
I call it the “magic moment.” It’s that moment in the scenario I described above, right before the doctor comes in. You’re sitting on the crinkly-paper exam room table (with your phone put away!). You look up and see a display of approachable, aspirational brochures. Or maybe it’s a mounted digital device that invites you to learn more about your symptoms with a few taps of your fingertips. The privacy of the exam room is a safety net where you can learn more about the reason for your visit, arm yourself with questions for your doctor and feel empowered to discuss your diagnosis after engaging with meaningful, condition-specific education.
What constitutes meaningful education? Easy-to-understand, for starters. Eighty-seven percent of U.S. adults read at an intermediate level or lower, so materials are most effective when written in what health literacy experts call “plain language” – a fifth-grade reading level, at most.[1] It’s easy for us as marketers to lose sight of this and go for the more complex messaging, but if patients can’t understand what we are saying, they won’t be empowered to ask the right questions.
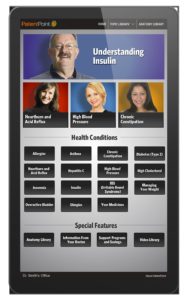 Then there’s the medium itself, which can also make patient education more meaningful: interactive touchscreen technology, engaging videos and, yes, even print – each medium is particularly effective for certain patient demographics. All serve as an opportunity to appeal to a patient’s learning preference and to provide a guide to a patient’s condition.
Then there’s the medium itself, which can also make patient education more meaningful: interactive touchscreen technology, engaging videos and, yes, even print – each medium is particularly effective for certain patient demographics. All serve as an opportunity to appeal to a patient’s learning preference and to provide a guide to a patient’s condition.
But content is also key. The most meaningful exam room education addresses not just the condition, but the underlying causes, lifestyle management and treatments as well. Treatment options will obviously vary depending on the condition and the progression of the disease state – so education must help patients learn how to communicate with their doctors throughout their diagnosis and journey.
This approach works. According to PatientPoint results, 9 out of 10 patients learned a tip they could take action on from our exam room education, while 96% of patients agreed we make health information easy to understand.[2] This translates into 5 to 1 ROI and 11% NRx average lift.[3]
So the next time you’re in your doctor’s office, sitting on that crinkly-paper exam room table, take a look around. Look for information that’s meaningful and draws you in. If you’re seeing one of the healthcare providers that such provides such resources, it’ll be there, ready to empower you to take control of YOUR health.
References
[1] Static Brain, “Illiteracy Statistics.” 2014.
[2] PatientPoint national survey. 2015.
[3] Symphony Health Solutions. 2015.



 For the last two years, we have conducted a revealing survey among women making healthcare decisions asking how much they trust the pharmaceutical industry – the WEST Survey on Pharma: Women’s Engagement, Satisfaction & Trust. This year, we also conducted the same survey among 300 men, the MENT Survey on Pharma: Men’s Engagement, Needs & Trust, again through our subsidiary Health Stories Project, an online community of people inspired to tell their health stories to help others to connect and learn.
For the last two years, we have conducted a revealing survey among women making healthcare decisions asking how much they trust the pharmaceutical industry – the WEST Survey on Pharma: Women’s Engagement, Satisfaction & Trust. This year, we also conducted the same survey among 300 men, the MENT Survey on Pharma: Men’s Engagement, Needs & Trust, again through our subsidiary Health Stories Project, an online community of people inspired to tell their health stories to help others to connect and learn.
 Advantages
Advantages Opportunities
Opportunities Benefits
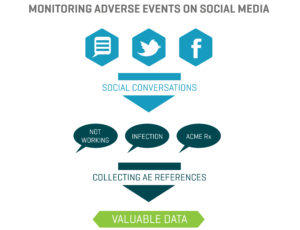
Benefits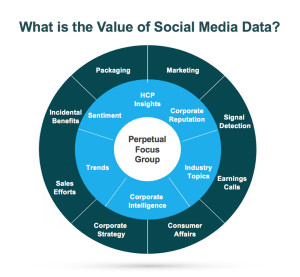 In the healthcare industry, patients and healthcare providers (HCPs) are looking to social media for information and support regarding their health and the health of their patients. They serve as a “perpetual focus group,” whose conversations taking place on social media provide brands with the opportunity to listen. Patients share their opinions, ask questions about diseases and treatment options, and directly or incidentally report adverse events. Meanwhile, HCPs voice opinions on the healthcare industry, provide thought leadership on a wide range of topics such as participatory medicine, reimbursement, and medical education. In a recent survey, more than 40 percent of respondents reported that information found via social media would affect the way they coped with a chronic condition or their approach to diet and exercise; 34 percent said it would affect taking certain medication.1 This demonstrates to companies that patients are directly impacted by what they view on social media and in order for brands to leverage that data radiating out of all the social conversations, several factors need to be in place.
In the healthcare industry, patients and healthcare providers (HCPs) are looking to social media for information and support regarding their health and the health of their patients. They serve as a “perpetual focus group,” whose conversations taking place on social media provide brands with the opportunity to listen. Patients share their opinions, ask questions about diseases and treatment options, and directly or incidentally report adverse events. Meanwhile, HCPs voice opinions on the healthcare industry, provide thought leadership on a wide range of topics such as participatory medicine, reimbursement, and medical education. In a recent survey, more than 40 percent of respondents reported that information found via social media would affect the way they coped with a chronic condition or their approach to diet and exercise; 34 percent said it would affect taking certain medication.1 This demonstrates to companies that patients are directly impacted by what they view on social media and in order for brands to leverage that data radiating out of all the social conversations, several factors need to be in place.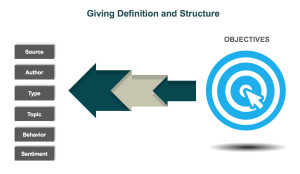 d is looking to identify who is talking about a product, the kinds of content they are posting, and the topics within their content, a structure that would classify posts into categories such as Consumer, Branded Product, and Medical Inquiry could be utilized. As more and more posts are tagged, patterns, trends, and themes can be identified.
d is looking to identify who is talking about a product, the kinds of content they are posting, and the topics within their content, a structure that would classify posts into categories such as Consumer, Branded Product, and Medical Inquiry could be utilized. As more and more posts are tagged, patterns, trends, and themes can be identified.
