In analyzing data from November and December for its sixth wave of research, the Commonwealth Fund, Phreesia, & Harvard found that, even though there was another surge of COVID-19 cases largely across the country, the number of outpatient practice visits remained stable. When compared to the baseline week of March 1, the November and December outpatient visits were “unchanged”.
The news release also highlighted that “several notable changes” were detected during that time period:
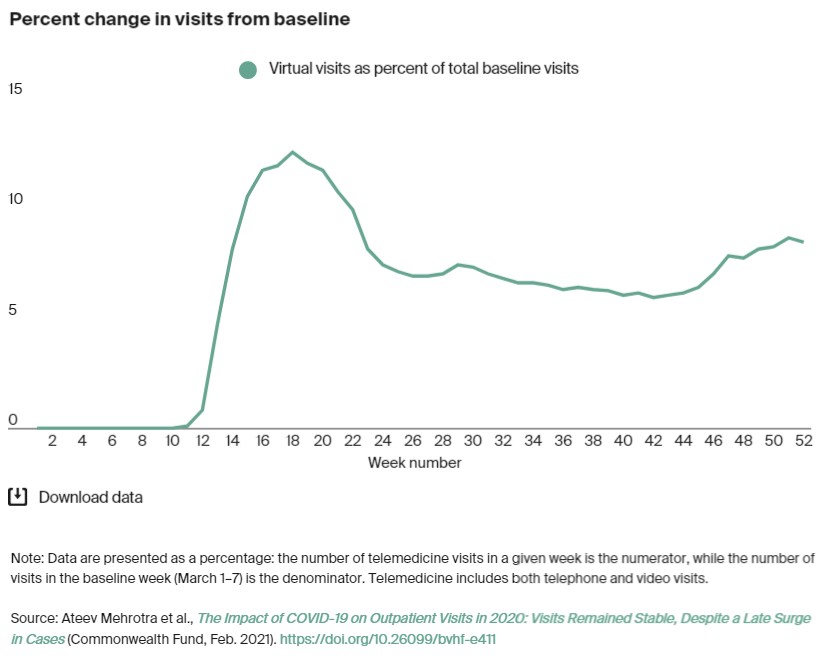
- Telemedicine experienced a modest increase of usage again. As seen in the chart below, just over 8% of visits were telemedicine visits.
- While those winter months typically see a rise in patient visits, “they were below the levels” in those two months of 2020; particularly among children, which showed a “substantial drop”. A typical-year shows a 7-10% increase above baseline for December visits among children ages 0-2. In December 2020, visits were 20-21% below baseline for that age group. A typical-year trend for December visits by children ages 3-5 shows a 6-10% increase above baseline. In December 2020, a 34-38% decrease was recorded for the age group. A typical-year trend for December visits by patients ages 6-17 range from 3% above baseline to 1% below baseline. In December 2020, an 18-25% below baseline was charted for the age group. Older age groups “remained more stable”.
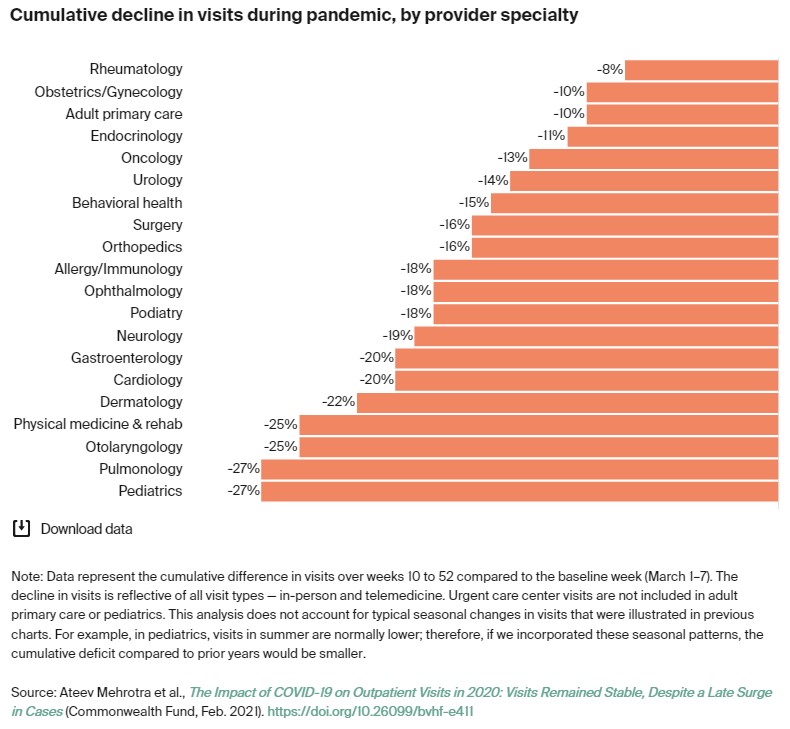
While all specialties saw declines during the pandemic, the report queried, “One critical question is whether visit volumes will rise above the baseline level as we gain increasing control over the pandemic and people receive care that had been deferred.”