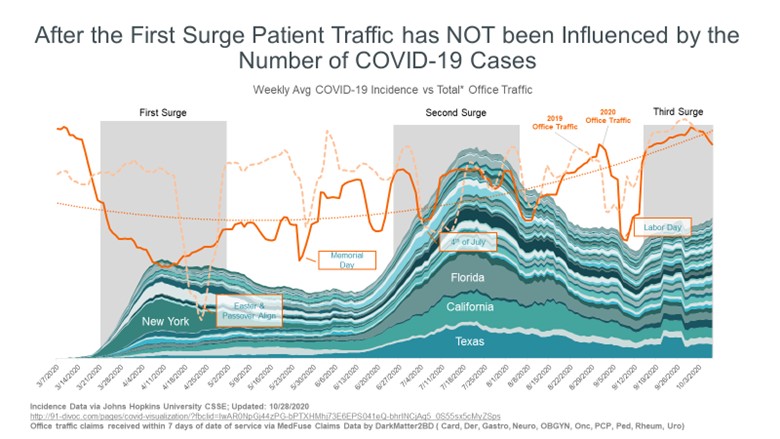
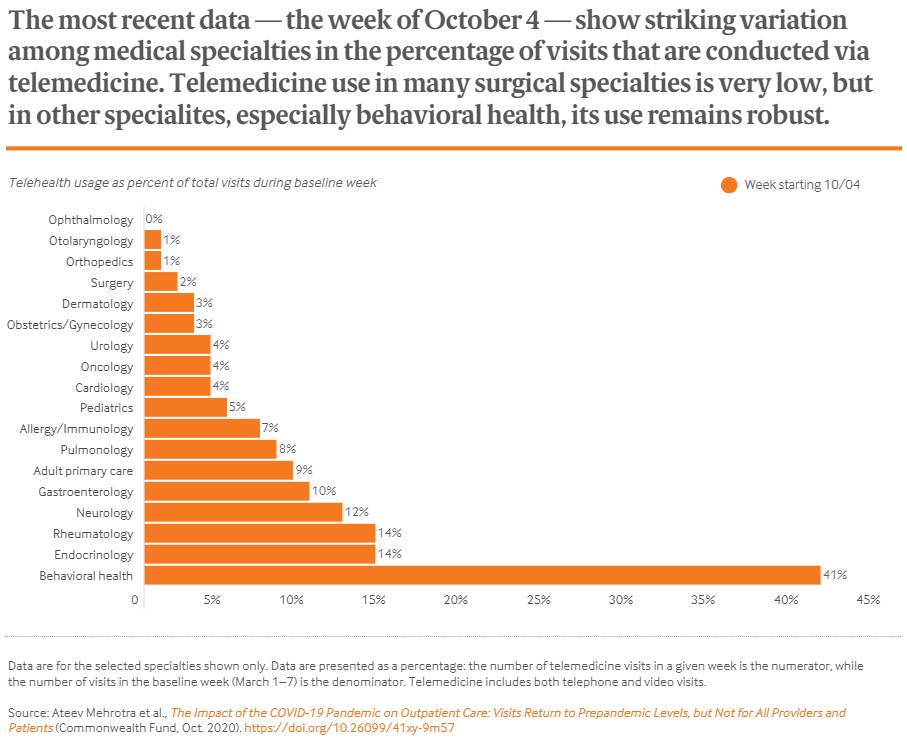
As part of President Biden's National Action Strategy to combat the global pandemic, he has instructed his COVID-19 Response Coordinator to work with the Department of Health and Human Services (HHS) in securing an additional 100 million doses of each of the two COVID-19 vaccines that have been approved by the FDA from Pfizer-BioNTech and Moderna. According to a White House news brief, this “increases the total vaccine order for the U.S. by 50%, from 400 million to 600 million with these additional doses expected to deliver this summer. With these additional doses, the U.S. will have enough vaccine to fully vaccinate 300 million Americans by the end of this summer.”
Moderna confirmed this news yesterday, stating that they are in talks with the US government, noting that the delivery will be in the third quarter of 2021. The previous amount purchased by the US government was 200 million doses of Moderna's and Pfizer-BioNTech's vaccinations each.
Last Friday, Pfizer and BioNTech had announced “an advance purchase agreement with COVAX for up to 40 million doses of the Pfizer-BioNTech COVID-19 Vaccine. The doses will be delivered throughout 2021.” COVAX is the collaboration of the Global Alliance for Vaccines and Immunization (GAVI), the Coalition for Epidemic Preparedness Innovations (CEPI), and the World Health Organization (WHO), to ensure fair global distribution of COVID-19 vaccines.